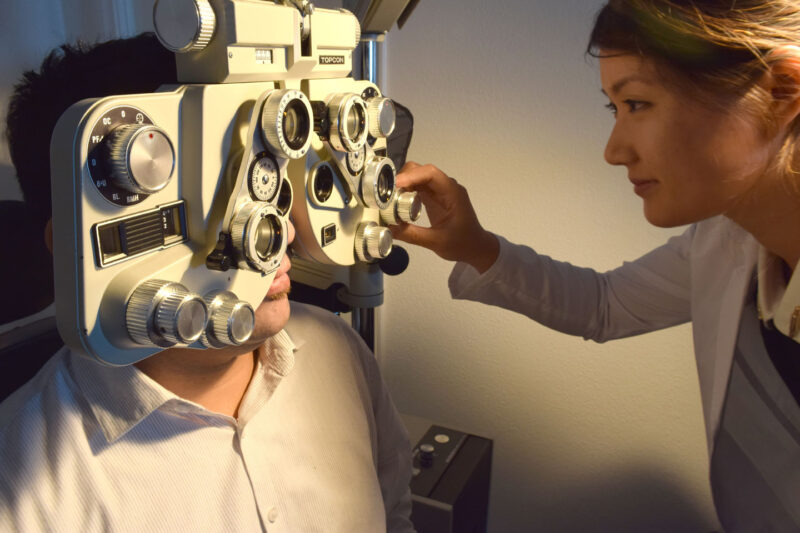
The MiSight® 1 day is a daily disposable contact lens specifically designed for myopia control. It is a centre distance dual focus concentric ring design with alternating distance vision correction zones (two) and treatment zones with +2.00D of defocus (two). It is NOT a multifocal contact lens in the traditional sense of those prescribed for presbyopia. Instead, MiSight 1 day is a dual-focus lens which is designed to create simultaneous defocus on the retina for both distance and near viewing1 – with an image falling on the retina to correct myopia and the +2.00D of defocus falling in front of the retina to create myopic defocus as a ‘slow-down’ signal for childhood eye growth.
MiSight 1 day was the first soft contact lens to be approved by the United States FDA for slowing the progression of myopia in children (see FDA indication footnote below).

Above is an image of MiSight® 1 day. The lens design features:
- Two treatment zones create myopic defocus with image focus in front of the retina, rather than behind it to slow axial elongation
- Two correction zones correct myopia in all gaze positions
For whom is MiSight 1 day suitable?
MiSight 1 day is a soft, daily disposable contact lens designed specifically for myopic children and was the first myopia control intervention to be granted a CE indication. In 2019, MiSight 1 day became the world’s first myopia management intervention to be FDA approved in the USA.
Initial fitting and visual outcomes with MiSight 1 day
This wide central distance zone provides excellent visual acuityand subjective vision outcomes, no different to an equivalent single vision contact lens,2 and simple first fitting based on the spherical equivalent refraction at the contact lens plane. A recent conference abstract3 presenting data from the MiSight 1 day three year clinical trial showed that almost 70% of MiSight 1 day fits require no over-refraction adjustment from their best-vision sphere starting power for trial lens fitting. The MiSight 1 day fitting guide suggests only adding extra minus if it improves distance vision.
Select the power of the MiSight 1 day trial lens to match the spherical equivalent refraction at the contact lens plane. Let this settle for at least 10 minutes to assess both lens fit and vision outcomes.
How is visual acuity with the MiSight 1 day?
The MiSight 1 day three year clinical trial found that best corrected acuity with MiSight 1 day was within one letter for the dual focus lens and the control (single vision) lens at distance and near, with a mean of around 0.00 logMAR (6/6 or 20/20) or better in both lens types.2
Distance and near best-corrected visual acuity should not be any different with MiSight 1 day, compared to a single vision contact lens correction.
Is any adaptation time required?
Some children may require an adaptation period to the dual-focus optical design of MiSight 1 day, when compared to a single vision contact lens. The MiSight 1 day fitting guide mentions that “under certain circumstances (such as low light levels), this optical design may cause reduced image contrast, symptoms of ghost images, and/or glare or halos around bright lights.”
Typically, these symptoms should be mild and should resolve within the first week or so of wear. It is best to explain to a child that their vision might feel ‘different’ rather than ‘blurred’ and that it should improve with time.
Does MiSight 1 day alter binocular vision function?
The answer is no – think of MiSight 1 day as functioning like a single vision contact lens in terms of its fitting and visual acuity outcomes, and in terms of binocular vision too. Because it is NOT a multifocal contact lens, it does NOT provide a near add as multifocal contact lenses do for presbyopes. Studies have shown that MiSight 1 day does not alter binocular vision function when compared to single vision spectacles4 or contact lenses.5,6
How well do the lenses fit?
The MiSight 1 day lens should prove a well-fitting lens for the majority of children. In the MiSight 1 day clinical study, only 6 out of 144 children showed unacceptable lens fit and did not proceed – three wearing MiSight 1 day and three wearing the control single vision lens.2
How do children find handling and comfort?
A survey undertaken on 8- to 12-year-old children who were new to CL wear found that after 1 week, 57% reported handling as ‘kind of easy’ or ‘really easy’. This increased to 85% at one month and 97% from 6 months through to the end of the 36 month study. Comfort was also reported highly, with over 95% reporting they ‘sometimes’ or ‘don’t notice’ the feeling of CLs on their eyes.7
Long term outcomes with MiSight 1 day
Myopia control
Randomized controlled trials indicate that children aged 8-12 years with -0.75 to -4.00D of myopia and no more than 0.75D of astigmatism, who wear MiSight 1 day contact lenses, are less likely to have rapid myopia progression, and are more likely to show refractive stability than their peers wearing single vision lenses.2,8
The MiSight 1 day three year clinical trial found that after 3 years, children wearing MiSight were 0.73D less myopic on average than children wearing the single vision contact lens.2
Over three years, 41% of MiSight wearing eyes showed no clinically meaningful change in spherical equivalent refraction (-0.25D or less change) in comparison to 4% of the single vision control lens wearing eyes. By contrast, 62% of the control eyes progressed by more than -1.00D compared to 18% of MiSight eyes.

Further data indicates that children fit at age 11-15 years may also benefit from a reduction in their myopia progression over three years, and that continued MiSight wear of up to six years appears to be beneficial for slowing myopia progression.9
Visual acuity and visual function
On follow up, best-corrected acuity should be 6/6 or 20/20 in the lenses at distance and near.2 MiSight also doesn’t influence accommodative or binocular vision function compared to single vision spectacles, or over time.3
Children report very high levels of satisfaction when wearing MiSight, with almost all giving a rating of seeing ‘really well’ or ‘kind of well’ for school. In the three year clinical trial, only 10% of MiSight 1 day wearers reported ‘slightly annoying’ visual disturbances like ghosting, halos or glare, compared to 5% of single vision Proclear® 1 day wearers, although this did not affect wearing time or continuation of wear throughout the study.7
Ocular health
As mentioned above, lens fit and comfort are normally without complication in children.2,7 Six years of MiSight and/or Proclear 1 day contact lens wear has been shown to have no significant impact on ocular health, with biomicroscopy signs being no different to pre-contact lens wear and no serious adverse events occurring.10
In evaluating safety in the community eye care setting, data from seven US eye care clinics was reviewed alongside that from randomized, controlled clinical trials (Ruiz-Pomeda et al 20188 and Chamberlain et al 20192 MiSight 1 day studies) for children aged 8-12 years at soft contact lens fitting. The rate of microbial keratitis was 7.4 per 10,000 years-of-wear and corneal inflammatory events 0.66% per year. This fills an important gap in understanding safety in young wearers, with rates no higher than that in adults wearing SCLs.11
Where does MiSight 1 day fit in your myopia control toolkit?
The MiSight 1 day dual focus contact lens has been demonstrated in two randomized clinical trials to be safe,2,8,10,11 effective2,8,9 and well accepted3,7 by myopic children. It has the longest dataset available of any myopia controlling soft contact lens, with results available to six years.9
The key advantages of the MiSight 1 day are that it is a daily disposable and has the longest and most robust data available on its safety and efficacy of any soft contact lens option. It is also the first and currently only myopia control intervention which has received United States FDA indication for myopia control.